The field of science has been blooming since the last few hundred years. Revolutionary theories have been proposed, and formulas have been discovered that helped change the world for the better.
It wouldn’t be an exaggeration to say that even small formulas like speed = distance/time have helped in bringing about technological development in the world.
One of these revolutionary discoveries is Avogadro’s Law. Examples are the best way to understand anything in science, in this article, we will learn or understand Avogadro’s Law Examples.
Also Read: What is Archimedes Law
Do you know who created Avogadro Law, how, and why? No, I tell you, The Italian Scientist Amedeo Avogadro introduced Avogadro’s Law in 1811.
This law is an experimental gas law. Specifically, this law is for an ideal gas. This law is not applicable to real gases because they show some variations. Understand Avogadro’s Law Examples, Ballons.
Table of Contents
What is Avogadro’s Law?
Avogadro’s law states that –
“At similar and constant physical conditions like Temperature and Pressure, the two different gases like hydrogen and nitrogen in the same volume contain an equal number of molecules.”
How was Avogadro’s Law Influential?
Avogadro’s law was able to explain how equal volumes of gases at ideal conditions gave an equal number of molecules. Although scientists at that time rejected this hypothesis, it gained acceptance after his death, when other scientists realized how important and revolutionary it was in the field of science. You can understand this law with Avogadro’s Law Examples, Lungs, Tyres, etc.
Mathematical Derivation of Avogadro’s Law
From the Ideal Gas Equation,
PV=nRT
Where, P = Pressure, T = Temperature, n = number of molecules of the gas, V = volume of the gas, R= Ideal gas constant (also known as Boltzmann Constant = 0.082057 L atm K-1 mol-1)
Assume P & T = Constant
V = n (RT/P)
Where, K = RT/P (Proportionality constant)
V α n
The above equation says that as the volume is increasing, the number of molecules of the gas is also increasing. Or As the volume decreases the number of molecules of the gas is also decreases (like in Ballons). Although Avogadro’s Law can be written in a lot of ways, this relation is the easiest to understand.
At condition 1 (at constant temperature and pressure),
At Condition 1, the Volume of gas is denoted as V1 and the number of molecules of a gas is denoted as n1.
At Condition 2 (P, T = Constant)
At Condition 2, the Volume of gas is denoted as V2, and the number of molecules of a gas is denoted as n2.
According to Avogadro’s law,
V1/n1 = V2/ n2 ————————————————————-(a)
Assume volume V2 is the twice of the volume V1, So,
V2= 2V1 ———————————————————————(b)
Put Value of V2 in equation number (a)
V1/n1 = 2V1 / n2
n2 = 2n1 ——————————————————————–(c)
According to equation (c ), If the number of volumes is doubled, then the number of molecules of a gas is also doubled.
Note: This law is for Ideal gases. In real gases, slight variations and deviations occur.
What is Avogadro’s number?
The Scientist Avogadro did not discover Avogadro’s number. The number of molecules in 1 mole is known as Avogadro’s number. Its value in the SI unit is 6.02214129 ×1023 mol-1. And it is denoted by NA. Nowadays, Avogadro’s constants are used instead of Avogadro’s number.
Avogadro’s Law Graph
Graphical representation of Avogadro’s law,
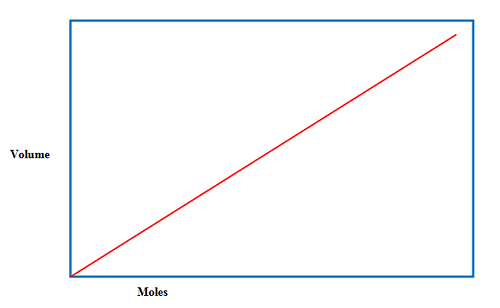
The above graph is plotted (shows linear relationship) between volume and mole at the constant physical condition. As the volume is increasing, then the molecules are also increasing.
Real-World Application of Avogadro’s Law
Since Avogadro’s law is for ideal gases, people think that it does not have any real-world Avogadro’s Law Examples and that it is just a hypothesis. But that is not the case. Avogadro’s law has numerous real-world applications, and it explains a lot of things that go on in our day to day life.
Lungs
The first real-world Avogadro’s law examples can be seen in our own body. We all know that the lungs help us in breathing. But have we ever wondered about the mechanism? Thanks to Avogadro’s law, it’s easier to understand how our Lungs work.
When we breathe in (inhale), air flows inside our lungs, causing the lungs to expand (increase in volume). From our lungs, the oxygen is extracted and injected into our bloodstream. Then, we breathe out (exhale), causing the Carbon Dioxide (CO2) to pass through, and thus shrinking the volume of the lungs.
Sports
All of us love to play games, right? No? Are you not into sports? It doesn’t matter, but if you’re looking for Avogadro’s law examples, you will find them in games like Football and Basketball. When you blow air into a ball, it causes the volume of the ball to increase, as air molecules start to collect inside of it. And if you puncture the ball, all that air will come out with pressure, causing the ball to shrink and create a loud sound. Avogadro’s law comes into play to explain to us all the things that are going on here.
Tires
Everyone found yourself in a situation where you got stuck in the middle of the road because of a flat tire? Well lucky if you haven’t. Things like puncture, temperature, or time can cause the tire to lose the air inside it, which decreases the volume of the tire and ruins your trip. When you take your car to the gas station, you also get the tires checked. When air is blown into the tires, they get inflated, and the volume is increased, and your car can move smoothly. This is another of Avogadro’s Law Examples.
Balloons
One more Avogadro’s Law Examples is a balloon. Everyone loves balloons. And to bring them to that beautiful state, you have to force air from your mouth into the balloon, causing it to expand to a much higher volume.
Limitations of Avogadro’s Law
Although Avogadro’s law has numerous real-world applications (Avogadro’s Law Examples discussed above) if it also has a lot of limitations. This doesn’t mean that Avogadro’s Law is not accurate; it just means that it is not perfect. So some of the limitations of Avogadro’s Law are listed below,
- The biggest limitation of Avogadro’s Law is that it was introduced to the world, taking into account that it works best for ideal gases. But such a thing as an ideal gas doesn’t exist in the real world. To get perfect and accurate answers, you have to apply this law to ideal gases. For real gases, this law still answers, but the accuracy is often questionable.
- Calculation of Heavy molecules tends to give poor and inaccurate results as compared to lighter gas molecules. This is where the limitation of Avogadro’s law is observed since the calculations for some major heavy molecules turn out to be slightly, or sometimes, mostly inaccurate.
- In real gases, temperature plays a significant role in increasing the ratio of volume to the mole as compared to ideal gases. This is because, in real gases, a temperature increase causes the gas to expand because of the intermolecular repulsion forces.
Avogadro’s law helps chemists and physicists in finding more theories and exploring new areas of chemistry.
